abbott point of care covid test
Abbott Laboratories ID NOW COVID-19 point-of-care test will be shipped to hospitals care clinics and doctors offices across the country starting Wednesday. Abbott Labs tops earnings estimates as COVID-19 test sales surge.
Laboratory Id Now Abbott Point Of Care Test Pcr Mobile Device Autodoc
How Covid-19 taught us the value of a transcendent laboratory service.

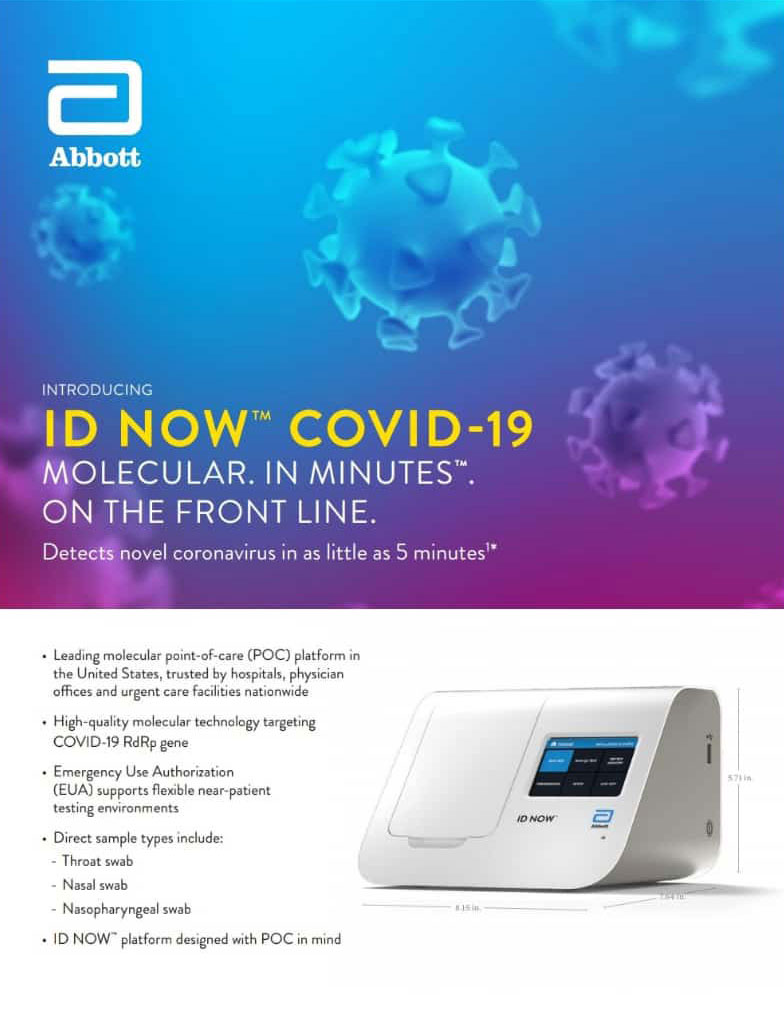
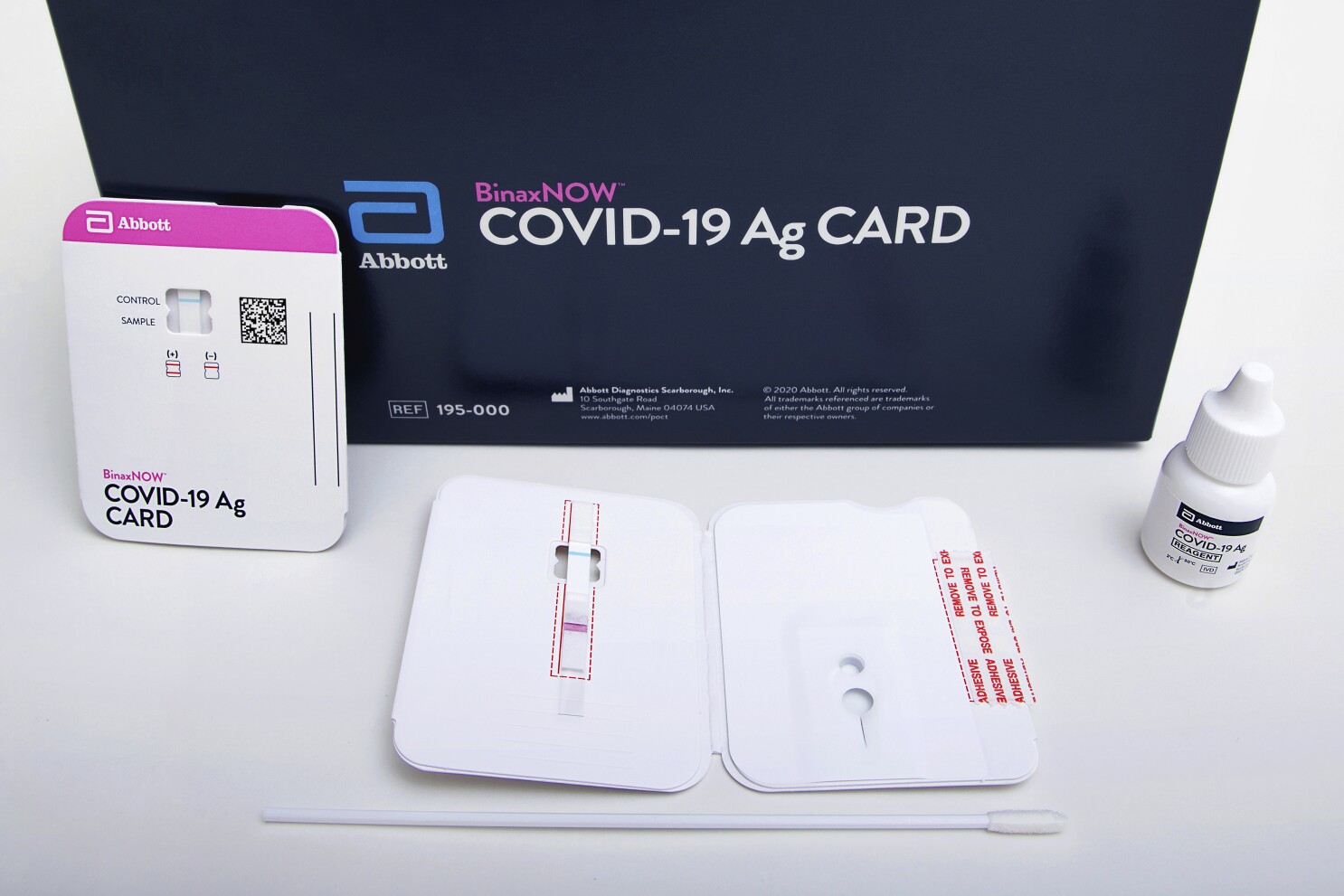
. Abbotts BinaxNOW COVID-19 Ag Card test can identify these antigens which are typically detected after symptoms start. Food and Drug Administration FDA for the fastest available molecular point-of-care test for the detection of novel coronavirus COVID-19 delivering positive results in as little as five minutes and negative results in 13 minutes. The Rhode Island Department of Health RIDOH strongly encourages facilities to test.
The tests are intended to identify the virus by recognizing a unique section of the coronavirus genome and amplifying that portion until theres enough for. Food and Drug Administration FDA for the ID NOW COVID-19 test in March 2020. To help provide the critical diagnostic information needed Abbott is currently providing and.
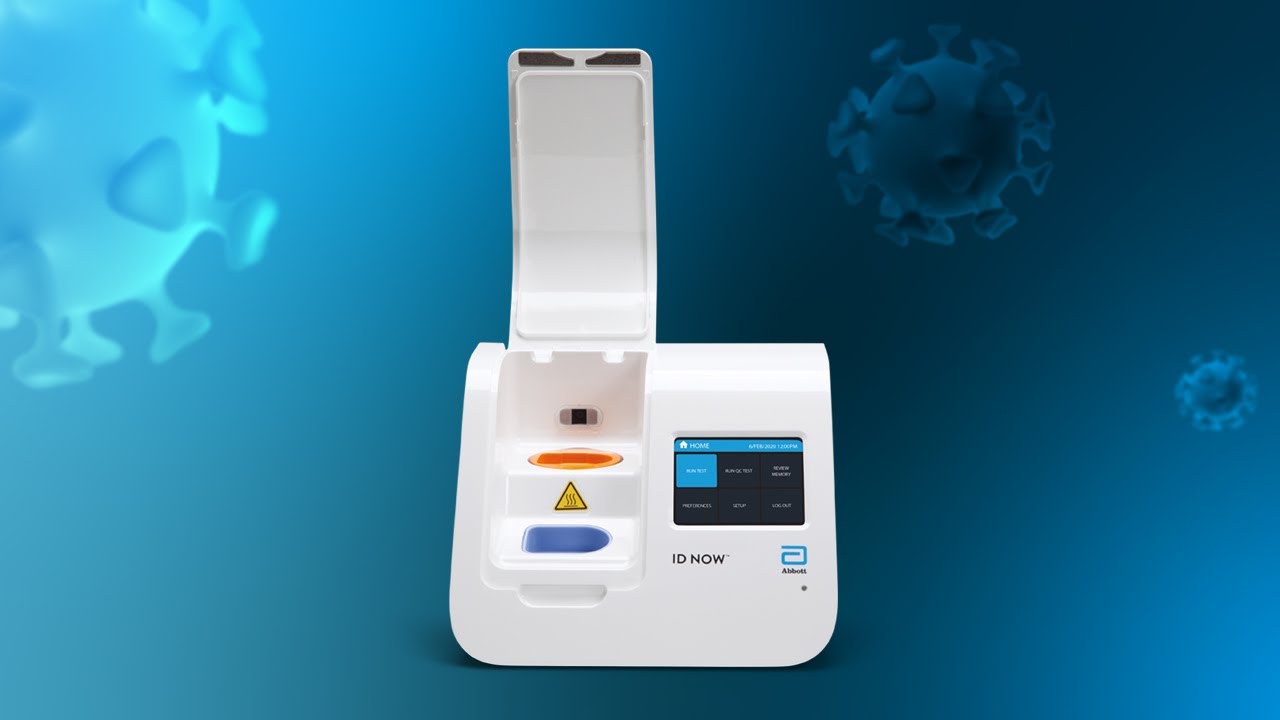
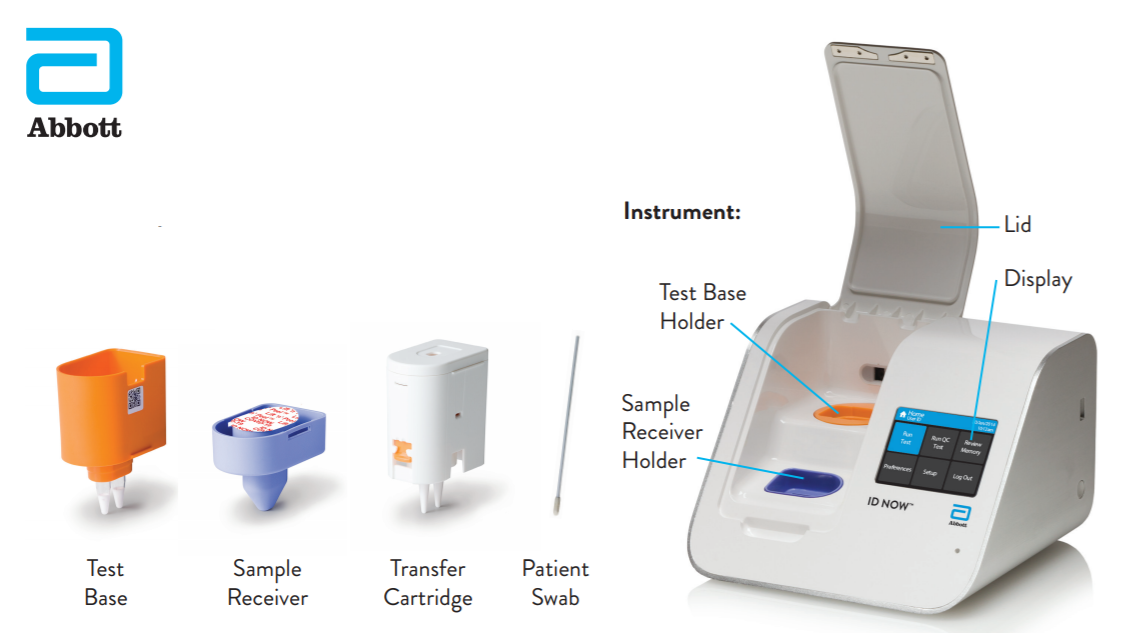
It is used on our ID NOW platform. ID NOW is an FDA approved CLIA-waived instrument which means that. Food and Drug Administration Emergency Use Authorization EUA.
COVID test sales were 33 billion in the quarter more than 90 of which came from our rapid test including BinaxNOW in the US Panbio internationally and ID NOW globally. The lab of the future is evolving. Republican Texas Gov.
Abbott has received emergency use authorization EUA from the US. A box containing a 5-minute test for COVID-19 from Abbott Laboratories is pictured during the daily briefing on the novel coronavirus in the. The company says it will ramp up its.
Driven in large part by. Nursing homes and assisted living facilities may use rapid antigen point-of-care POC tests to test personnel residents or visitors for COVID-19. The lab of the future.
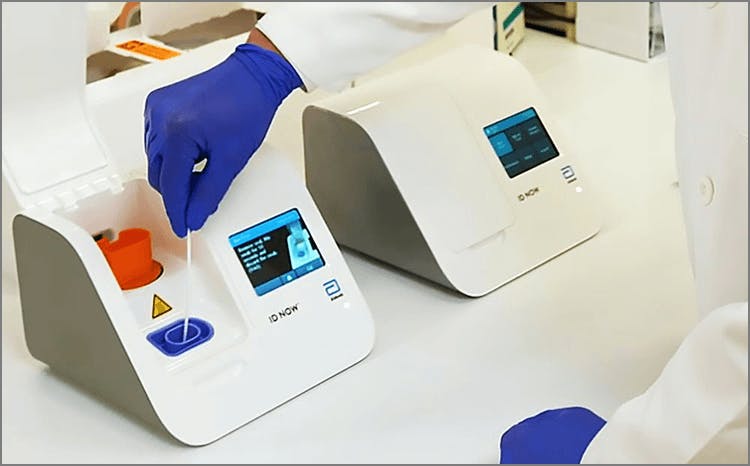
Results from the simple nasal swab are available in 15 minutes through testing individuals suspected of COVID-19. 128 10 05. The portable rapid molecular ID NOW COVID-19 test has emerged as a critical part of this arsenal allowing fast diagnosis with results in 13 minutes or less in a variety of locations such as physicians offices urgent care clinics and other point-of-care locations.
Apr 20 2022 900 am. CLIA-certified laboratories or testing sites are no longer required to report negative results for non-NAAT. 23 rd of April 1215 1315 CET Hall L Pavilion 4 Lunch will be served.
The revolutionary NAVICA app helps people navigate daily life in a new normal. The Food and Drug Administration FDA has issued an Emergency Use Authorization for the Abbott ID Now COVID-19 test a molecular point-of-care test that delivers results within minutes allowing healthcare professionals to make clinical decisions during a patient visit. Second quarter of 2021 for a restructuring plan related to Abbotts manufacturing network for COVID-19 diagnostic tests.
The COVID-19 pandemic is affecting all of us around the world. Greg Abbotts short-lived policy of requiring state troopers to conduct secondary inspections of trucks crossing into Texas from Mexico cost the United States almost 9 billion in just 10 days Axios reported Tuesday. 2 days agoABT earnings call for the period ending March 31 2022.
Reporting Requirements for Rapid Testing in Point-of-Care Settings. Abbott positioned to be leading COVID-19 point-of-care diagnostics manufacturer in the US says GlobalData Posted in Pharma Abbotts rapid COVID-19 antigen self-test the BinaxNOW is now widely available across the US at CVS Pharmacy Walgreens and Walmart without a prescription. Abbotts molecular point-of-care test for COVID-19 delivers positive results in as little as five minues and negative results in 13 minutes.
The policy which Abbott enacted on April 6 snarled truck traffic at the border and led to a protest by Mexican truckers that. 2 days agoPoint of Care. Point of Care Diagnostics sales rose 04 organically.
NAVICA displays results from the 15-minute Abbott BinaxNOW COVID-19 Ag Card rapid antigen test to help individuals make informed decisions. As a leader in diagnostic testing we have a unique responsibility to contribute our expertise to help fight the COVID-19 pandemic. A CLIA-certified laboratory or testing site must report all positive SARS-CoV-2 diagnostic and screening test results to the person who was tested or that persons healthcare provider.
The tests can be used in point-of-care settings and at home with an online service provided by eMed. With healthcare systems changing the demands to the lab are more complex and innovative tools and solutions are available to meet new demands and excel in delivering lab. The ID NOW COVID-19 test is a rapid molecular point-of-care test that detects COVID-19 in 13 minutes or less.
Abbott Laboratories ABT 171 Q1 2022 Earnings Call. Personnel and residents who. Personnel and residents who have symptoms of COVID-19 regardless of their vaccination status.
The Abbott ID NOW COVID-19 test brings rapid testing to a wide range of front-line healthcare environments such as physicians offices urgent care clinics and hospital emergency departments. Abbott received emergency use authorization EUA from the US. Abbott has rapid point-of-care solutions to support your COVID-19 and influenza testing needs ID NOW COVID-19 The ID NOW COVID-19 assay is now available for use on the ID NOW platform under US.
BinaxNOW COVID-19 Ag Card has received US. Find out more about this innovative technology and its impact here. According to Abbott the rapid test which runs on the ID NOW platform is an.
Abbott is putting its resources towards helping you navigate this crisis. What makes this test so different is where it can be used. Food and Drug Administration Emergency Use Authorization EUA.
Minutes Not Hours Rapid Testing For Coronavirus Youtube

Laboratory Id Now Abbott Point Of Care Test Pcr Mobile Device Autodoc
/cdn.vox-cdn.com/uploads/chorus_asset/file/19856029/IDNOW_INACTION3_macro_300dpi_1200x628.jpg)
A New Covid 19 Test Can Return Results In 5 Minutes The Verge

Abbott Id Now Covid 19 Instructions Modified

Abbott Id Now Covid 19 Detection Test System Us

14 000 Rapid Covid 19 Testing Kits Coming To Grey Bruce Ctv News

Demand For Abbott Labs Covid 19 Tests Soars Past 40 Million As Pandemic Cases Surge

Indonesia Go Id Cartridge Nya Isi Ulang Diagnosisnya Lima Menit
Instant Results From Abbotts Covid 19

Laboratory Id Now Abbott Point Of Care Test Pcr Mobile Device Autodoc
Covid 19 Rapid Molecular Swab Test Good Doctors Medical Centre
Fda Authorizes Covid 19 Test That Doesn T Need Special Equipment Los Angeles Times

Rapid Test Bioquick Abbott Nasal Rapid Test Antigen Covid 19 Autodoc

As Problems Grow With Abbott S Fast Covid Test Fda Standards Are Under Fire Kaiser Health News

Steps To Use Id Now Effectively Abbott Newsroom

Panbio Covid 19 Ag Rapid Test Device Abbott Point Of Care

Abbott Labs Rapid 5 Covid 19 Test To Fill In Testing Gaps For Millions In The U S
